On July 25, 2018 The European Court of Justice (ECJ) ruled that crops and food created using gene-editing techniques such as CRISPR will be subject to the same regulations as those governing genetically modified organisms, including the 2001 directive which imposes high hurdles for developing GM crops for food. The decision is likely to hinder investment in crop research using CRISPR in the EU. The ASEAN, especially Thailand and Malaysia, is one of the world’s center for vegetable research and development. Seeds companies and developers want to avoid the problems of communication and politics that occurred with GMOs, including tensions between organic and GMOs, consumer rejection of many varieties of GM foods, increased number of food companies avoiding GM ingredients. In this article we are reviewing whether laws of Thailand and Malaysia on GMOs labelling should apply to genome-editing techniques (“CRISPR”).
CRISP has proved to be a promising development in various fields especially in agriculture, by genetically altering specific types of cells. Compared to other techniques used to insert, delete or replace DNA in the genome of an organism, CRISPR is faster, easier to use and cheaper, potentially more precise in its application, and can also be used to edit multiple genes simultaneously. The international regulatory landscape on GMOs separates countries into three types
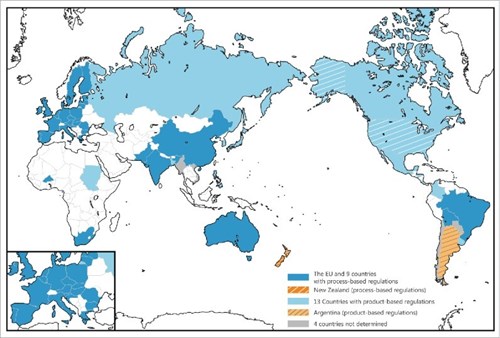
The blue countries in the map below have adopted process-based GMO regulations. The light blue countries have employed process-based GMO regulations. The USA (product-based GMO regulations), Argentina (product-based GMO regulations) and New Zealand (process-based GMO regulations) are highlighted with stripes because they have already made the regulatory responded to genome-edited crops.
Thailand
The Notification of Ministry of Public Health (No. 251) B.E. 2545 applies to food obtained through certain techniques of GMOs. Under Article 1 of the Notification, soybean and soybean products, corn and corn products, obtained through certain techniques of genetic modification/genetic engineering shall be subjected to labelling.
Article 2 further states that food under Clause 1 means soybean and soybean products, corn and corn products which contain “recombinant DNA or protein resulting from gene technology from 5 % up of each top three main ingredients in terms of the ratio of weight they occupy, and each weight ratio accounts for five or more % of the total.” From the above, CRISPR should be exempted from GMO labelling.
Malaysia
The relevant legislation is the Bio-safety Act of 2007 and guidelines on the release of GMOs into the Environment.
"modern biotechnology" is defined in the Bio-safety Act as the application of - (a) in vitro nucleic acid techniques, including recombinant deoxyribonucleic acid (DNA) and direct injection of the nucleic acid into cells or organelles; or (b) fusion of cells beyond the taxonomic family, that overcome natural physiological reproductive or recombination barriers and that are not techniques used in traditional breeding and selection.
"products of such organisms" means any product derived from a living modified organism or part of a living modified organism(a) if the product contains detectable recombinant deoxyribonucleic acid (DNA); or (b) where the profile, characteristic or properties of the product is or are no longer equivalent to its conventional counterpart irrespective of the presence of the recombinant deoxyribonucleic acid (DNA);
Unlike in Thailand, the legislation on GMOs is likely to apply to CRISPR.
These two examples suggest that countries throughout the world are divided regarding their policies toward genome-edited crops. Some countries deregulate transgene-free crops, whereas others regulate all types of crops modified by genome editing. Prior to launching CRISPR products on the market, it is critical to consider whether GMO’s related laws should apply.